Atmospheric pressure is an indicator of how much coffee you will drink today. And on a more serious note, changes in this pressure can significantly affect our mood. For us divers, it is also an essential part of dive planning. Today you will learn everything you need to know about atmospheric pressure in simple terms.
Did you sleep a bit in physics classes at school and don’t quite remember what all the pressure was about? Don’t worry, I wasn’t the best student in the class either. But now, as a scuba diving instructor, I can explain to my students what it’s all about. After reading this text, you will understand atmospheric pressure in simple terms and how it affects your dives. Shall we begin?
What Is Atmospheric Pressure?
Let’s start with the basics: what exactly is atmospheric pressure? As you read this text, your lungs are constantly filling up with air because we need it to live. This air, although invisible at first glance, is composed of various particles. And it all has its own weight. The air weighs something, even if we can’t see it.
When air presses on any surface with its weight, this is what we call atmospheric pressure. Think of it this way: we live at the bottom of an ocean of air. Just like a fish at the bottom of the sea has water pressing down on it from above, we have air pressing down on us from above.
In other words, this pressure refers to the force exerted by the Earth’s atmosphere on a given surface. It’s the weight of the air above that surface, caused by Earth’s gravity pulling on air molecules. Simple, isn’t it?
Did you know? The entire atmosphere weighs about 5.5 quadrillion tons, roughly one-millionth of the Earth’s mass. That means there are about 15 pounds pushing down on every square inch of your body right now, which is roughly the weight of a car pressing down on you all the time!
Why Divers Need to Understand Atmospheric Pressure
You might be wondering why this matters for diving. Well, atmospheric pressure is the starting point for understanding all pressure changes underwater. When you descend, you’re adding water pressure on top of atmospheric pressure. Your body, your scuba equipment, and the air you breathe all respond to these pressure changes. Understanding atmospheric pressure helps you make sense of depth gauges, dive tables, and why certain safety rules exist.
How Do We Measure Atmospheric Pressure?
To talk about the impact of pressure on our lives, we first need to measure it somehow. Fortunately, this is something that has been around for a long time. The first pressure measuring device, called a barometer, was built by Evangelista Torricelli in 1643.
There are different types of barometers, but the three most commonly used are the mercury barometer, the aneroid barometer, and the modern digital barometer.

Mercury Barometer
This type of barometer consists of a long glass tube that is closed at one end and filled with mercury. The open end of the tube is submerged in a container of mercury. Atmospheric pressure exerts pressure on the mercury in the container, causing it to rise up the tube.
The height of the mercury column is a measure of pressure. Standard atmospheric pressure at sea level can support a column of mercury about 760 millimeters high, which is known as one standard atmosphere. This empty space at the top of the tube is called the Torricellian vacuum, named after its inventor.
Aneroid Barometer
Unlike a mercury barometer, an aneroid barometer does not use a liquid. Instead, it uses a small, flexible metal box called an aneroid cell. This cell is sensitive to changes in atmospheric pressure. As the pressure changes, the cell expands or contracts, which then translates into a corresponding movement of the needle on the dial. The dial is calibrated to provide a pressure reading.
The aneroid barometer is more portable than the mercury version, which makes it more practical for everyday use and for carrying in the field.
Digital Barometer
Nowadays, modern digital barometers are also available that use electronic sensors to measure atmospheric pressure. Their advantage is that they provide accurate and instantaneous readings of atmospheric pressure. You probably have one in your smartphone right now without even knowing it.

Atmospheric Pressure Measurement Scales
Okay, so we already know what we can measure atmospheric pressure with, and now we need to adopt some common measurement scale. Here, unfortunately, as always, several measurement scales have been created and I understand that they can cause confusion. So let’s see what we measure this pressure in.
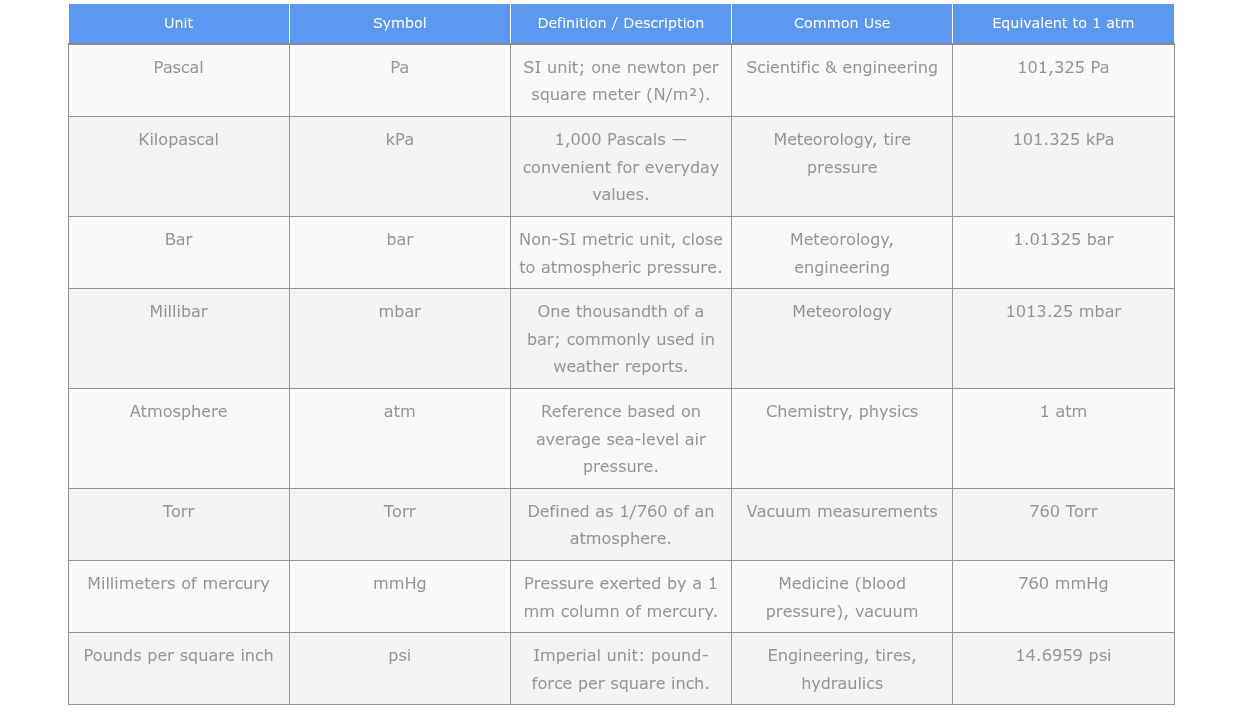
Common Pressure Units Explained
Pascal: The pascal is the SI unit of pressure. One pascal is equal to one newton per square meter. Atmospheric pressure is typically measured in kilopascals or hectopascals, where 1 kPa equals 1,000 Pa and 1 hPa equals 100 Pa. Standard atmospheric pressure at sea level is 101.325 kPa or 1,013.25 hPa.
Bar: The bar is a unit of pressure derived from the metric system. One bar is equal to 100,000 pascals. Atmospheric pressure is often reported in millibars, where 1 mb equals 1 hPa. At sea level, standard atmospheric pressure is approximately 1.013 bar.
Millimeters of Mercury: This scale is commonly used in meteorology and aviation. It represents the height of a column of mercury that the atmospheric pressure can support. The standard atmospheric pressure at sea level is approximately 760 mmHg.
Inches of Mercury: This scale is similar to mmHg but uses inches as the unit of measurement. One inch of mercury is equivalent to approximately 25.4 mmHg. The standard atmospheric pressure at sea level is approximately 29.92 inHg. You’ll hear this measurement on weather reports in the United States.
Atmosphere: The atmosphere is a unit of pressure representing the average atmospheric pressure at sea level. One atmosphere is approximately equal to 101,325 pascals, 1,013.25 millibars, 760 mmHg, or 29.92 inHg. It’s also equal to 14.7 pounds per square inch.
Which Units Do Divers Use?
As you can see, we have several different scales of measurement and this can cause some confusion. The different scales have a slightly different purpose, but when it comes to measuring pressure in scuba diving we mostly use BAR or ATM (atmosphere). These scales are used to determine the pressure of air as well as water.
In the United States, you’ll also frequently see PSI (pounds per square inch) used for tank pressure. Your submersible pressure gauge probably reads in PSI if you dive in the U.S., or in BAR if you dive elsewhere in the world. Standard atmospheric pressure at sea level is 14.7 PSI.
Understanding Absolute vs. Gauge Pressure
Here’s where things get interesting for divers. When we talk about pressure, we need to distinguish between two different ways of measuring it: absolute pressure and gauge pressure. This distinction is crucial for understanding your dive gauges and dive physics.
What Is Gauge Pressure?
Gauge pressure is pressure measured relative to atmospheric pressure. In other words, gauge pressure uses atmospheric pressure as its zero point. This is the type of pressure reading you see on most of your dive equipment.
For example, when your submersible pressure gauge reads zero, your scuba tank isn’t actually empty of all air. It still contains air at atmospheric pressure (14.7 PSI or 1 bar). The gauge just ignores that atmospheric pressure and only measures pressure beyond it. This gives you a reading of usable pressure, which is what you actually care about when planning your dive.
Your depth gauge works the same way. At the surface, it reads zero even though you’re experiencing 1 atmosphere of pressure from the air above you. As you descend, it only measures the additional pressure from the water.
Another everyday example is tire pressure. When your car tire says it needs 32 PSI, that’s 32 PSI gauge pressure, not including the atmospheric pressure that’s already there.
What Is Absolute Pressure?
Absolute pressure is the total pressure, including atmospheric pressure. It’s measured relative to a perfect vacuum (zero pressure). When you’re underwater, absolute pressure is the combination of atmospheric pressure plus water pressure.
This is the type of pressure used in dive physics calculations, particularly when working with Boyle’s Law or other gas laws. The formula is simple:
Absolute Pressure = Atmospheric Pressure + Water Pressure
At sea level, atmospheric pressure is 1 atmosphere. At 10 meters depth in saltwater, you have 1 atmosphere from the water plus 1 atmosphere from the air above, giving you 2 atmospheres absolute pressure.
Fun fact: A completely empty vessel at sea level would show approximately negative 14.7 PSI on a gauge pressure meter, but it would show 0 PSIA on an absolute pressure meter. That’s because gauge pressure can go negative (below atmospheric), but absolute pressure can never go below zero.
Ambient Pressure in Diving
You might also hear the term ambient pressure. This simply means surrounding pressure. On land, ambient pressure comes from the weight of the atmosphere. At depth, it comes from the weight of the water plus the weight of the atmosphere. Ambient pressure can be expressed as either absolute or gauge pressure depending on the context.
How Atmospheric Pressure Changes
Atmospheric pressure is not constant everywhere. It changes with altitude, weather conditions, and even time of day to a small extent.
Altitude and Atmospheric Pressure
The higher you go, the lower the pressure. This happens because there is less air above you pressing down. Conversely, the closer you are to sea level, the higher the pressure.
Surely you are familiar with the fact that climbers in high mountains have oxygen cylinders so that they can breathe fairly normally. This is due to the low pressure at high altitudes. Low pressure leads to lower air density, and thus less oxygen per liter of air. So with each breath you take in less oxygen, which you need to live.
At sea level, atmospheric pressure is about 14.7 PSI or 1 bar. But if you climb to an altitude of 5,000 feet like Denver, Colorado, the atmospheric pressure drops to about 12.2 PSI. This is why altitude diving requires special procedures and tables, because the baseline atmospheric pressure is different.

Weather and Atmospheric Pressure
But altitude is not the only thing affecting the change in atmospheric pressure. It can also vary due to factors such as weather conditions and time fluctuations. Atmospheric pressures are also heavily influenced by unique weather events like storm fronts or hurricanes.
High pressure systems typically bring clear, calm weather. Low pressure systems often bring clouds, wind, and precipitation. Meteorologists use barometers to track these pressure changes and predict weather patterns. In general, pressure shows fluctuations due to many factors and can affect your mood, among other things.
Atmospheric Pressure and Your Mood
It has been suggested that atmospheric pressure can potentially affect your mood. Some individuals may experience changes in mood or physiological effects in response to changes in pressure, although scientific evidence is limited and inconclusive.
It is thought that changes in atmospheric pressure, especially abrupt or significant ones, can affect the balance of certain chemicals and neurotransmitters in the brain, potentially leading to changes in mood. Some people say they experience symptoms such as headaches, fatigue, irritability or changes in sleep patterns during periods of fluctuating pressure, such as before a weather change. That is, something like jet lag after a long trip.
However, it is important to remember that the relationship between atmospheric pressure and mood is highly individual and not universally experienced. Many factors, including personal sensitivity, pre-existing health conditions and psychological factors, can also affect mood. If you feel such an effect related to the change in atmospheric pressure, just have a cup of good coffee.

Pressure Changes and Scuba Diving
While the impact of a change in pressure on your mood can be very individual, the impact of pressure changes on divers is a serious aspect of dive safety. Most diving problems or accidents are precisely related to the change in pressure.
Why Pressure Matters Underwater
Of course, water pressure is a topic for another text, but I must point out something very important here. During diving, our body is exposed to increased pressure. And this is a significant difference. Water is approximately 800 times denser than air, so pressure increases much more rapidly underwater than it does when you climb a mountain.
This increased pressure leads to the absorption of nitrogen, which is part of the air we breathe. Since our body does not have the ability to process this nitrogen, it accumulates in our tissues. After diving, it will be removed over time, but this process requires the atmospheric pressure we have at sea level, which is 1 atmosphere.
The Danger of Rapid Pressure Changes
If we reduce this atmospheric pressure too quickly after a dive, the accumulated nitrogen will also be released, but much too quickly. This can lead to nitrogen bubbles forming in your blood and tissues, which in turn can lead to serious health consequences known as decompression sickness or DCS.
From this follows a very simple rule that every diver must know: after a dive it is a bad idea to fly in an airplane. You need to wait at least 12 to 24 hours (depending on your dive profile) before exposing yourself to reduced atmospheric pressure. Your body needs that standard sea level atmospheric pressure to safely off-gas the nitrogen you absorbed during your dive. Learn more about what you should never do right after diving.
Pressure at Depth: Salt Water vs. Fresh Water
Understanding how pressure increases with depth is fundamental to dive planning. The rate at which pressure increases depends on whether you’re diving in salt water or fresh water, because salt water has a different density than freshwater.
In saltwater: Pressure increases by 1 atmosphere for every 10 meters (or 33 feet) of depth.
In freshwater: Pressure increases by 1 atmosphere for every 10.3 meters (or 34 feet) of depth.
This means that at 10 meters depth in saltwater, you’re experiencing 2 atmospheres of absolute pressure: 1 atmosphere from the air above the water, plus 1 atmosphere from the 10 meters of water itself.
Here’s a simple table showing how pressure increases with depth in saltwater:
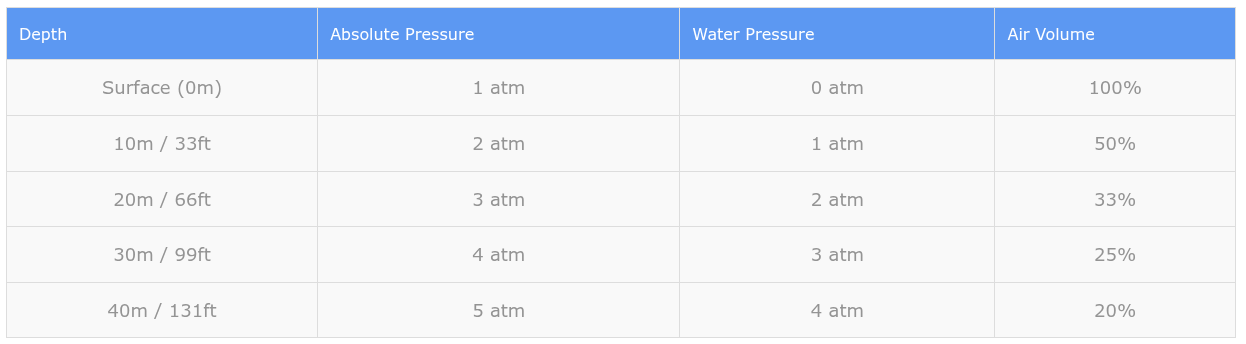
Notice how air volume decreases as pressure increases. This is Boyle’s Law in action, which states that as pressure doubles, volume is cut in half (assuming temperature remains constant).
Did you know? The greatest pressure changes per meter occur in the first 10 meters of depth. Going from the surface to 10 meters doubles the pressure, but going from 20 meters to 30 meters only increases pressure by 33%. This is why shallow water can be the most dangerous part of a dive for pressure-related injuries.
Atmospheric Pressure – Let’s Recap
I hope that if you had any doubts about what exactly atmospheric pressure is, you no longer have them now. Let’s quickly review what we’ve covered:
Atmospheric pressure is the weight of air pressing down on us, caused by gravity pulling on air molecules. At sea level, this pressure is approximately 14.7 PSI, 1 bar, or 1 atmosphere. We measure it using barometers, which were first invented by Torricelli in 1643.
As you can see, we are constantly subjected to various forces and changes beyond our control. Atmospheric pressure changes with altitude and weather, and it can even affect our mood. But most importantly for divers, atmospheric pressure is the baseline from which all underwater pressure calculations begin.
Pressure during a dive
When you dive, you add water pressure on top of atmospheric pressure to get absolute pressure. Understanding the difference between gauge pressure and absolute pressure helps you read your equipment correctly and plan safe dives. And remember, after diving you need to stay at sea level atmospheric pressure long enough for your body to safely release absorbed nitrogen.
Remember that diving without a basic knowledge of these relations will be at least dangerous, and certainly not very wise. If you want to learn about the underwater world, sign up for a scuba diving course and learn what every diver should know.
So that you not only look, but also speak like a diver.
Frequently Asked Questions About Atmospheric Pressure
What is atmospheric pressure in simple terms?
Atmospheric pressure is the weight of air pressing down on any surface. Think of it as living at the bottom of an ocean of air. At sea level, this pressure equals about 14.7 PSI or 1 bar, which is roughly the weight of a car pressing down on every square inch of your body.
How does atmospheric pressure affect scuba diving?
Atmospheric pressure is the starting point for all underwater pressure calculations. When you dive, water pressure adds to atmospheric pressure to create absolute pressure. This affects how much nitrogen your body absorbs, how your equipment reads pressure, and why you need to wait before flying after diving.
What is the difference between gauge pressure and absolute pressure?
Gauge pressure uses atmospheric pressure as its zero point and only measures pressure beyond it. Your dive gauges (SPG, depth gauge) read gauge pressure. Absolute pressure includes atmospheric pressure and represents total pressure. At 10 meters depth in saltwater, gauge pressure is 1 atm, but absolute pressure is 2 atm (1 from water + 1 from air).
Why can’t I fly immediately after diving?
During a dive, your body absorbs nitrogen due to increased pressure. After diving, your body needs sea level atmospheric pressure (1 atm) to safely release this nitrogen over time. Flying or going to high altitude reduces atmospheric pressure, causing nitrogen to be released too quickly, which can lead to decompression sickness. Wait at least 12-24 hours after diving before flying.
How is atmospheric pressure measured?
Atmospheric pressure is measured using barometers. The three main types are mercury barometers (measure mercury column height), aneroid barometers (use a flexible metal cell), and digital barometers (use electronic sensors). Modern smartphones often have built-in digital barometers.
Does atmospheric pressure change with weather?
Yes, atmospheric pressure fluctuates with weather systems. High pressure systems typically bring clear, calm weather, while low pressure systems bring clouds, wind, and precipitation. Storm fronts and hurricanes can cause significant pressure changes. Some people report experiencing headaches or mood changes during these pressure fluctuations.
What pressure units do divers need to know?
Divers primarily use BAR and ATM (atmospheres) to measure both air and water pressure. In the United States, PSI (pounds per square inch) is commonly used for tank pressure. At sea level, 1 atmosphere equals 1.013 bar or 14.7 PSI. These units help divers calculate depth, air consumption, and dive planning.
Sources and References
This article is based on verified information from credible sources in diving education, atmospheric science, and physics:
- NOAA – Ocean Physics and Atmospheric Pressure – Officialdata from the National Oceanic and Atmospheric Administration
- PADI Open Water Diver Course Materials – Professional dive training standards and pressure physics
- Encyclopedia Britannica – Atmospheric Pressure – Historical and scientific reference on pressure measurement
- NIST – SI Units for Pressure – Official pressure unit standards and conversions





